How To Neutralize Formaldehyde Gas Using Hydrogen Pero
How to avoid formaldehyde gas hazards indoors. A formula and method for rapid neutralization of formaldehyde solutions and vapor is provided.

The Hitchhiker S Guide To Hydrogen Peroxide Fumigation Part 2 Verifying And Validating Hydrogen Peroxide Fumigation Cycles Applied Biosafety
Atrophaeus biological indicators demonstrate the effectiveness of formaldehyde in decontamination of Camfi l HEPA fi lters.
How to neutralize formaldehyde gas using hydrogen pero. I was hoping if I had another chemical that would neutralize the H2S then I could fill a fire extinguisher with it and use that to make the air non-toxic. 1a Formalex GREEN should never be used to directly treat concentrated 37. Most of adoptable methods which are used for the safeguard of operators health cant frequently avoid situations where.
Test pH of the spill after the neutralization reaction has stopped with pH paper 4. Formaldehyde is neutralized by reaction with sulfite ion where the sulfite ion is provided by dissolving a sulfite-containing compound or neutralizer such as sodium sulfite sodium bisulfite or. Formalin solutions often contain some amount of methanol 10-15 vv as a stabilizer.
This is usually done using ammonia created by heating ammonium bicarbonate or ammonium carbonate. Initially the pH of the formaldehyde solution will be about 6. Formaldehyde Spill Cleanup VIII.
Formaldehyde Safe Use Practices VII. Drain all aerosol canisters. Be careful not to over-neutralize 3.
The invention comprises a matrix or medium upon which a mixture of urea acid calcium chloride and water is applied and dried. Safer might be Neutralization of Formaldehyde Gas by Ammonium Bicarbonate and Ammonium Carbonate which would greatly reduce the flammability of the product. Use of hydrogen peroxide to neutralize formalin vapors during tissue sampling.
Decontamination of rooms and equipment with gas-eous hydrogen peroxide is used increasingly especially in. - I mean that perhaps not only formaldehyde was disposed of through the online tubingbut perhaps also eg. Slowly add acetic acid to a container of cold water to form a 110 dilution of acid to water.
Formaldehyde Waste Collection Disposal and Treatment Formaldehyde Template SOP Formaldehyde Online Training I. List of sources of formaldehyde gas or odors in buildings. Fifty percent of world methanol production is used for this purpose and much or most of this methanol is obtained by the steam reforming of natural gas followed by methanol synthesis from the resulting carbon monoxide and hydrogen.
Our photo left shows bare particle board visible on the under-side of a game table. Formaldehyde is produced commercially by the oxidation or dehydrogenation of methanol. After the formaldehyde contact time the formaldehyde gas must be neutralized.
The formaldehyde dehydrogenation to convert formaldehyde to formic acid38 By using a catalyst the formic acid product is very easily decomposed to hydrogen at near-room tempera-ture131525263940 In the meantime NAD is converted to NADH by the hydrogen deposited from formaldehyde. FORMALDEHYDE SAFETY GUIDELINES Formaldehyde is a colorless strong-smelling gas often found in aqueous solutions called formalin solution with a maximum concentration of 37-40. Possible to remove hydrogen sulfide and mercaptans using reagents-neutralizers.
1 10 formalin 4 formaldehyde and 4 glutaraldehyde are the highest concentrations that can be neutralized. Hydrogen Peroxide General Hydrogen Peroxide Vapor Decontamination Process Summary. BSC chamber to create formaldehyde gas which is circulated through the BSC for 6-12 hours.
Yes as the title suggests I need to neutralize some H2S so that I can safely enter some confined spaces. Reaction may produce heat. Hydrogen peroxide vapor is generated by evaporation or aerosolization of concentrated 30 35 aqueous hydrogen.
This will generate 31 g of hexamine approximately a 3 solution. Neutralizing H2S-- Rendering Hydrogen Sulfide in the air into a non-toxic state. Nitric acid or HCl - it might be difficult to tell about the real chemical reaction.
How to reduce formaldehyde exposure levels in a buildingrmaldehyde gas odors or hazards in buildings. Cheney and Collins 1995. Return pool chemicals back to your local pool store.
In a biodegradation experiments of a wastewater sample containing formaldehyde ranging from 315 to 125mglresidual formalin a solution of formaldehyde gas in water ranging from 40 to 85 respectivelywas found at the end of the run 16d showing the inhibition effect of formalin which increased with the. The problem of formaldehyde exhalation during tissue sampling for histological examinations is deeply felt in each histological laboratory. At the same time neither the reagent itself nor the reaction products should be corrosive and degrade the quality of the raw material.
Store solvents for special collection. When the treated matrix or medium comes into contact with formaldehyde solution or vapor the hydroscopic nature of the calcium chloride dried on the medium quickly absorbs. Method 2 Disposing of Common Chemicals Use proper safety precautions.
While you may be able to clean the spill without protection if none is available it again may be better to call in a team to avoid acid exposure depending on your level of training. INTRODUCTION In its purest form formaldehyde is a colorless highly toxic and flammable gas with a strong pungent odor. When using neutralizers a chemical interaction occurs which results in the formation of inert low-toxic compounds 14-19.
Flush down the drain with an excess of cold water. Wait until bubblingfizzing has stopped a. Formaldehyde vapours leave precipitations especially when neutralized by ammonia that have to be and cleaned by hand which overall might be more harmful to surfaces and technical equipment Dietz et al.
Pour Amphomag slowly and directly onto the spill to contain and neutralize. Neutralize spill with a DILUTE acid such as vinegar 3M HCl citric acid 2. Ammonia water AKA ammonium hydroxide N H X 4 O H reacts with formaldehyde to form hexamine a comparatively innocuous solid though it is flammable when dry.
Slowly add a 1M solution of sodium hydroxide or sodium carbonate until the pH is in the range of 60 to 80. When using a neutralizing spill kit the kits are buffered and will not have a bubbling action. Researchers have for.
Dilute bleach and hydrogen peroxide to pour down the drain. As ammonia is added and stirred a fluffy white precipitate will result. To 1000 ml of 10 formalin 4 formaldehyde add 56 ml of strong ammonia solution 27.
Note that hydrogen gas will be produced in the process if conditions are alkaline and the formaldehyde. Formaldehyde can be oxidised to carbon dioxide under acid conditions by hydrogen peroxide. How to get rid of indoor formaldehyde gas.
Fixative solutions labeled as 10 buffered formalin are actually.
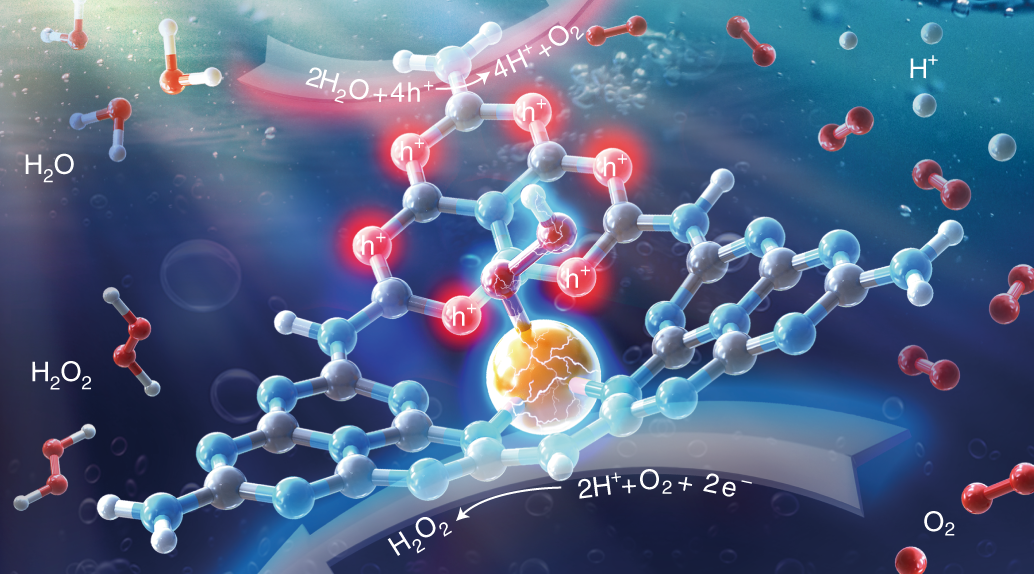
Atomically Dispersed Antimony On Carbon Nitride For The Artificial Photosynthesis Of Hydrogen Peroxide Nature Catalysis

Highly Efficient Photosynthesis Of Hydrogen Peroxide In Ambient Conditions Pnas
Comments
Post a Comment